Liquid Silicone Rubber, abbreviated as LSR in English, is a product favored by both consumers and manufacturers. Because of its excellent properties such as being non-toxic, odorless, corrosion-resistant, and biocompatible, it is highly favored in the medical field. Such as the breathing masks and infusion tubes that we often know are representative products of liquid silicone in medicine.
Table of Contents
ToggleAnalysis and Description of the Liquid Silicone Medical Market
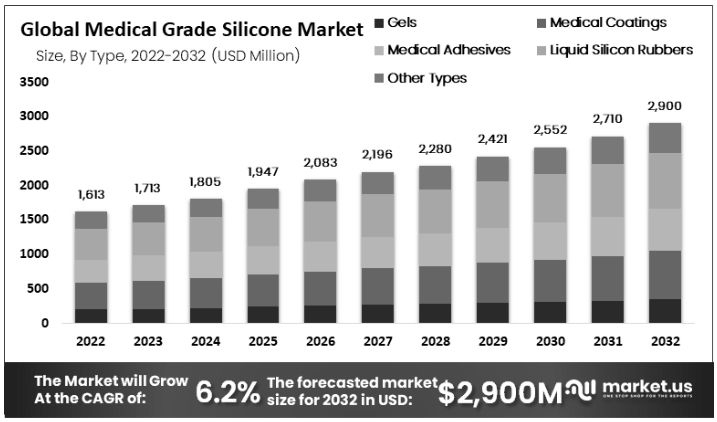
The global medical-grade silicone market size has reached 1.713 billion US dollars in 2023. During the forecast period from 2023 to 2032, the compound annual growth rate is 6.20%. It is expected that by 2032, the global medical-grade silicone market size will reach approximately 2.9 billion US dollars. According to the product form, the global medical-grade silicone market size is divided into gels, medical coatings, medical adhesives, liquid silicone rubber, and other products. Because it has better biocompatibility, high temperature resistance, chemical resistance, and bacterial resistance, liquid silicone is expected to obtain the largest market share. Liquid rubber is expected to occupy the largest market share, reaching 28%.
1.The Advantages of Choosing Liquid Silicone
Medical-grade liquid silicone (LSR) is a type of silicone material that meets strict quality and safety standards and can be used in the healthcare field. It is commonly used in the manufacturing of medical devices, implants, and components. Medical-grade liquid silicone is composed of bio-inert materials that comply with standards such as ISO 10993, USP Class VI, and RoHS. Medical-grade liquid silicone is temperature-resistant and easy to disinfect. In addition, it also has excellent stability and water resistance. Medical devices produced using medical-grade liquid silicone rubber (LSR) contribute to extended lifespans. The basic property of liquid silicone, biocompatibility, is particularly outstanding in the medical field. Liquid silicone is highly compatible with human tissues and has superior inhibitory effects on the growth and reproduction of bacteria. It is also extremely flexible and has a special self-adhesive property. Because it can alleviate pain, it is also widely used in the manufacturing of artificial joints and pacemakers. The growing demand for implantable devices made of medical-grade silicone is significantly driving the growth of the medical-grade liquid silicone market.
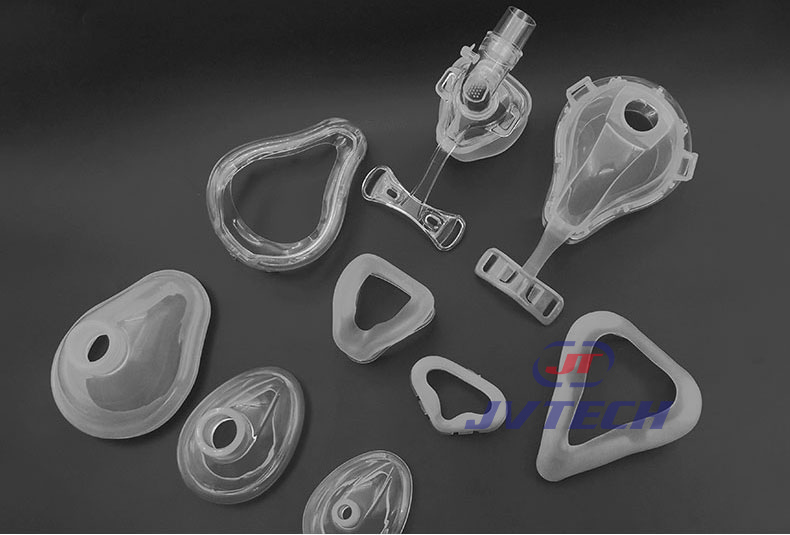
2.Application Display of Liquid Silicone Medical Products
2.1 Medical Components
Blood circuit components / Respiratory tubing components / Check valve components / Dental components / Ophthalmic components / Anti-slip components for medical devices / Electronic medical components / Silicone masks…

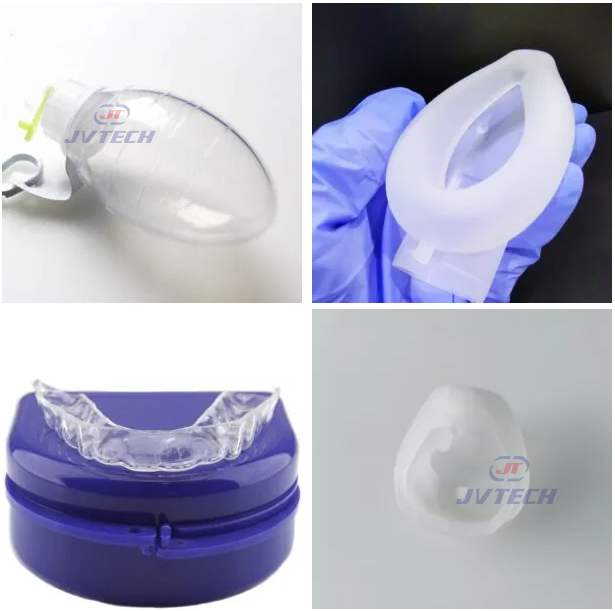
2.2 Medical Products
Drainage balls / Silicone breathing balls / Silicone breathing bags / CPR portable kits / Laryngeal masks / Two-piece silicone gastric tubes / Orthodontic dental molds…
3.Conclusion
Driven by the growing demand of the aging population (aged 65 or above), the rising prevalence of chronic diseases, the improvement of infrastructure and technological progress, the increasing demand for advanced medical services is expected to drive the growth of the global medical device industry, and then is expected to stimulate the demand for medical-grade silicone. In addition, due to the surge in the number of road traffic accidents and the increasing number of patients, the demand for surgeries is expected to be stimulated, which may further increase the demand for medical-grade silicone used in surgical instruments. Compared with high consistency silicone rubber (HCR), liquid silicone is more suitable for the medical market because it provides medical device manufacturers with shorter curing time, lower curing temperature and higher consistency. All these mean that the medical-grade liquid silicone market has a wide range of applications, stronger applicability, and a broad development prospect that can be foreseen in the future.





